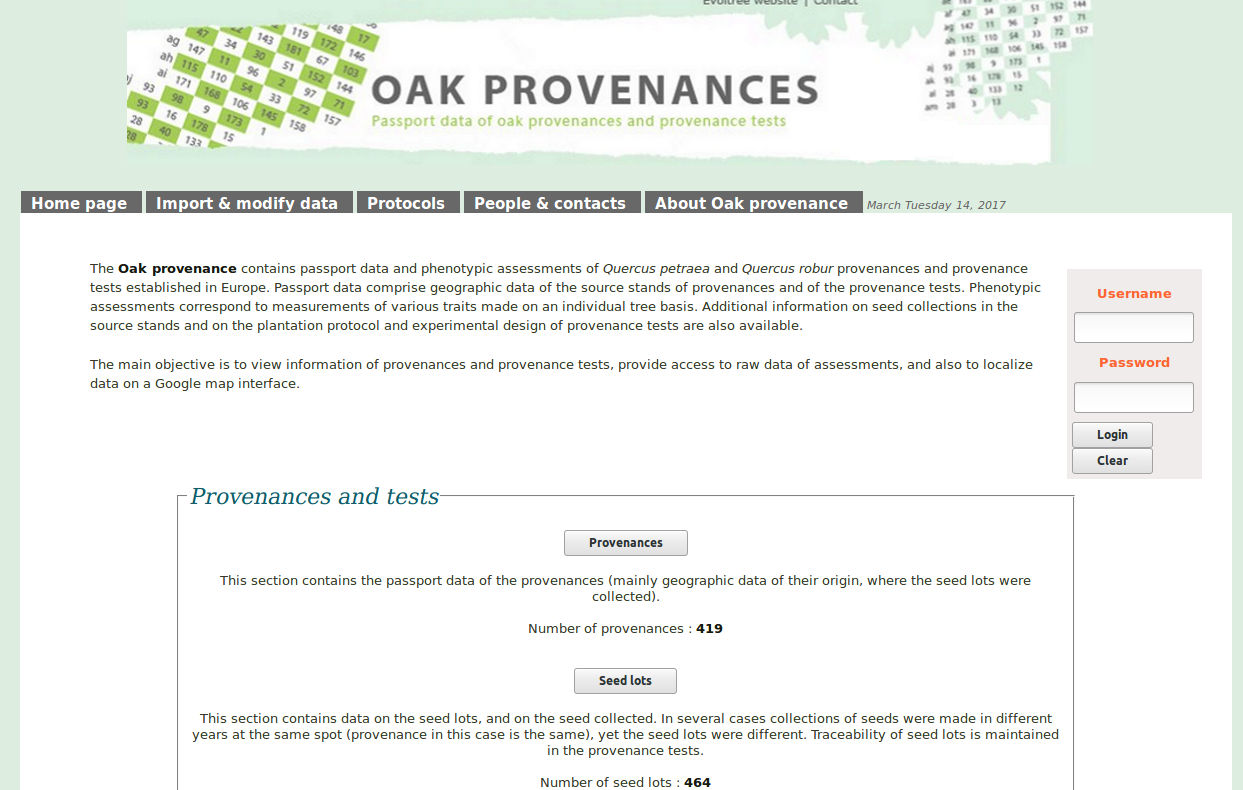
EVOLUTIONARY BIOLOGY
Origin and diversification
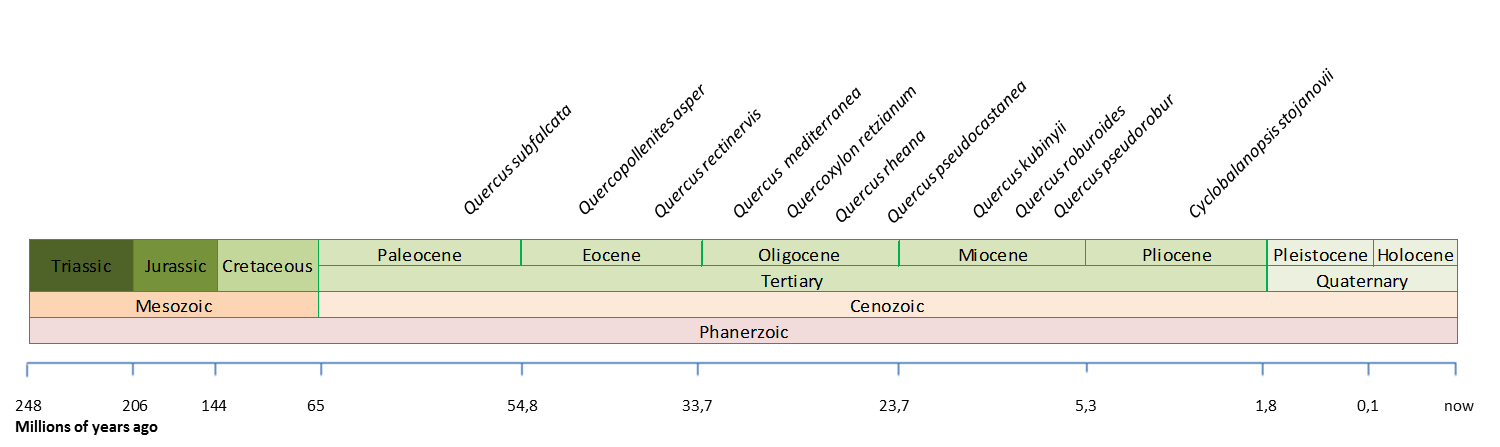
Fossil remains of oaks are common and have been discovered on the three continents. At deeper time scales, a rich fossil record supports the notion that the oaks have been around for at least 55 million years. The earliest occurrence of Oaks (Quercus) in the fossil record is in the Paleocene. The pollen grains of Quercoidites in the Late Paleocene (ca. 55 Ma) at the St Pankraz site (Austria) are currently the earliest evidence of the genus (Hofman et al., 2011). And some of the earliest remains found in North America (Bones, 1979; Manchester, 1994), China (Jiang, 1993), and Europe (Kvaček & Walther, 1989) are from the Eocene. A rich variety of palynological data indicate that sections Quercus, Lobatae and Protobalanus as well as the group Ilex were already present in middle Eocene Arctic localities. However, pre-Paleogene, and perhaps pre-Eocene occurrences of Quercus macroremains are generally represented by poorly preserved fossils that lack critical features needed for certain identification and need to be treated with caution (Barron et al., 2017).
The almost simultaneous appearance of the genus on the three continents, as witnessed by today’s detected fossil remains, has raised the question of the geographic origin and radiation of the genus. Two scenarios were proposed to reconstruct the history of the species. In the first scenario (Zhou, 1992), the genus appeared in South East Asia deriving from a sister genus Trigonobalanus during the Palaeocene, and migrated into two directions: to Europe and America via the North Atlantic Land Brigde (NALB) before the Eocene, and via the Bering strait after the Miocene.
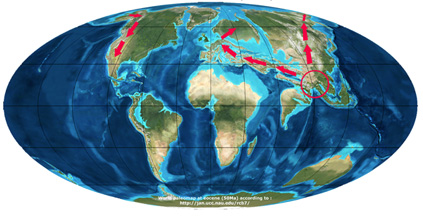 Scenario of Zhou (1992)
Scenario of Zhou (1992)
In the second scenario (Manos and Stanford, 2001 and Trelease, 1924), the genus Quercus derived from the widely distributed boreal-tropical deciduous forest that extended throughout the northern hemisphere at the beginning of the Tertiary. The genus further differentiated as the separation between the continents became more pronounced. As a result oak species occurred on the different continents as a vicariance between Asia and North America of an ancestral group composing the boreal-tropical forest.
- In Asia the “ancestral group” differentiated into the sub genus Cyclobalanopsis and the section Cerris of the genus Quercus. And species of the Cerris group migrated later westwards to Europe.
- In America, the “ancestral group” differentiated into section Lepidobalanus (white oaks), section Protobalanus and section Erythrobalanus (red oaks) with subsequent migration of white oaks from North America to Asia and further Europe.
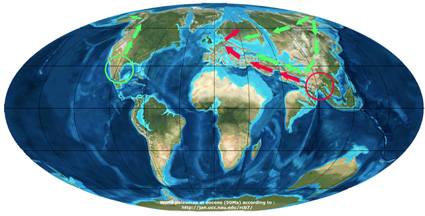 Scenario of Manos et al. (2001)
Scenario of Manos et al. (2001)
Hubert et al. (2014) suggested migration via the North Atlantic Land Bridge (NALB) and Beringia until the latest Pliocene of even Pleistocene interglacial. And although the fossil record does not contradict the North Atlantic connection, it suggests that the Beringian route was probably closed by 12 Ma (Barron et al., 2017). Previously, Manos and Stanford (2001) suggested a divergence time of 17 Ma between North American and Eurasian white oaks, which is relatively in agreement with the prevailing view of an early (Oligocene) termination of the NALB as a corridor for plants and animals. Yet, this is inconsistent with the general low genetic differentiation between North American and Eurasian members of white oaks (Denk and Grimm, 2010).
Overall, the combined evidence from modern phylogenetic studies and the fossil record (paleobotanical and phylogeographic results) suggests that the NALB acted as a transatlantic corridor for oaks until the Late Miocene (Barron et al., 2017). And in any case, the potential importance of the land bridge for the migration of the genus Quercus between Europe and North America during the earlier Cenozoic needs to be evaluated.
Despite the disagreement on the origin of the genus, paleobotanists agree on the extremely rapid diversification of the genus during the Oligocene and Miocene as a response to important climatic changes. Most fossil remains of that period are similar to extant samples. Hence it is believed that most of the extant species existed already at the mid Miocene (Trelease, 1924; Axelrod,1983).
Fossil records
EuropeThe genus Quercus is clearly present in Europe at the Paleocene–Eocene transition (ca.55 Ma) at St. Pankraz (Austria; Hofmann et al., 2011). One of the few late Paleocene sites that can be verified is that of Ménat, France. Here leaves have been assigned to Q. lonchitis Unger, Q. parceserrata, Q. provectifolia Saporta or Quercus subfalcata (Barron et al., 2017). In the Eocene, fossils from Quercus are well widespread in Europe. Wood remains occur from the beginning of the Eocene and belong mostly to the fossil genus of Quercoxylon (earliest evidences in Germany) (Gregory et al., 2009)
The earliest evidence of the group Ilex in Europe come from pollen grains found in the opencast mine at Cospuden (Germany) in the Eocene Quercus species like, Q. rectinervis and Q. olafsenii were common in the floras of EuroAsia in the mid-late Eocene playing an important role in the ecosystems. From the Oligocene to the middle Miocene, Quercus rhenana (possibly section Lobatae) was a dominant species in many places and was scattered through Central Europe inhabiting river-banks and swampy areas (Barron et al., 2017)
Species of the Cerris group (eg. Q. kubinyii) are an important element that inhabited European floras from the early Miocene and Neogene. One of the most relevant oaks in Europe from the middle Miocene to the early Pliocene is Q. pseudocastanea widespreading throughout Russia, Armenia, Kazashstan and western Siberia. Similarly Q. mediterranea is a typical floral component during the Neogene in all Europe widespread pre- in Europe appearing in the late Oligocene. In the Pre-Mediterranean vegetation of central and south Europe, Sclerophyllous evergreen oaks (sections Cerris and Ilex) were important elements during the Neogene. Some significant species for this period were Q. gigas, Q. pseudocastanea, Q. kubinyii, Q. mediterranea and Q. drymeja. The section Cyclobalanopsis is also present in Europe (as C. stojanovii) during the Pliocene (Barron et al., 2017).
In North Africa the earliest wood remains assigned to the genus Quercus are from the Oligocene age, especially Quercoxylon retzianum from the Petrified Forest of Cairo (El-Saadawi et al., 2011). Some of the most remarkable occurrence during the early Pliocene is the ring-cupped oak, Cyclobalanopsis stojanovii in Bulgaria (Beli Brjag Basin). From the late Miocene on the most frequent species in general in Europe were Q. pseudorobur and Q. roburoides (Barron et al., 2017).
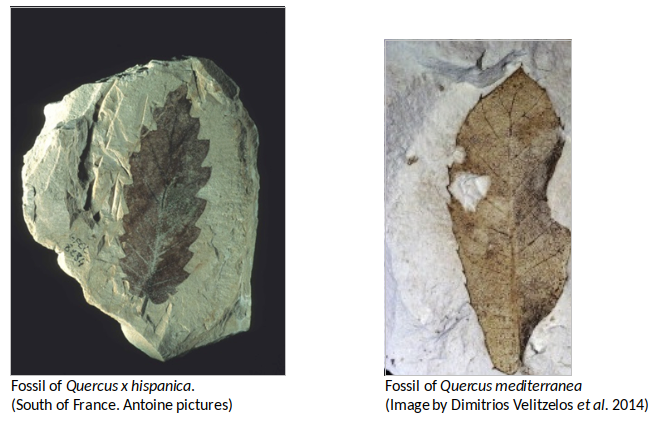
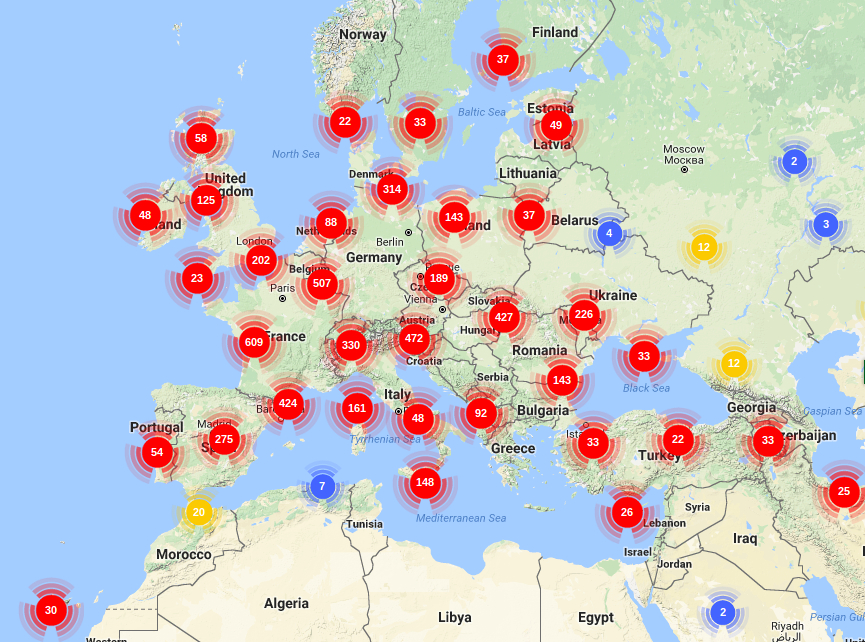
Most important fossils found so far of the Quercus genus in Europa (Barron et al., 2017; Xu et al., 2014; Binh et al., 2018. Illustration by Zamira Betancourt).
Asia
One of the first records of a Quercus type wood is Quercus cretaceoxylon from the upper Cretaceous in Japan (Suzuki and Ohba, 1991). Likewise the first occurrence of Quercus as a genus is from the early Paleocene, Quercus tsagajanica, in Pojark, Russia (Barron et al., 2017). Fossil leaves, acorns, and wood of oaks subgenus Cyclobalanopsis are reported from Japan and China as early as the Eocene, like Cyclobanalopsis naitoi in Japan (late-middle Eocene). The fossil record from Asian countries from the Oligocene is scarce, one of the most relevant is Quercus protoserrata, which is similar with modern Quercus serrata, and it was widely distributed throughout East Asia (Barron et al., 2017). Recently, Xu et al. (2016) found many leaf fossils of ring-cupped oaks in late Miocene strata of Mangkang County, eastern Tibet at 3910m.a.s.l. belonging to Q. tibetensis (where no extant ring-cupped oaks survive today), which indicates that Cyclobalanopsis oaks existed during the late Miocene in the core area of the Qinghai–Tibetan Plateau and suggests that the climate conditions in southeastern Qinghai–Tibetan Plateau during the late Miocene were likely warmer and more humid than today. The estimated paleo-elevation of the fossil site of Q. tibetensis during the late Miocene was at least 160 m lower than the modern elevation, indicating the continued uplift of eastern Tibet since the late Miocene. This uplift of the southeastern Qinghai–Tibetan Plateau is documented by dramatic vegetation changes in the Neogene: the late Miocene Kajun flora is dominated by Quercus subgenus Cyclobalanopsis, whereas the late Pliocene flora is rich in oaks section Ilex, which usually occurs in subalpine or alpine regions of southwestern China. (Xu et al., 2014).
From the early Oligocene, group Cerris diversified in Eurasia; however, it became extinct in North America by the Neogene. On the other hand, representatives of the subgenus Cyclobalanopsis ( = Cerris, Denk et al., 2017) disappeared from North America probably by the late Paleogene, and Europe at the end of the Neogene. Now, this subgenus is present only in East Asia.
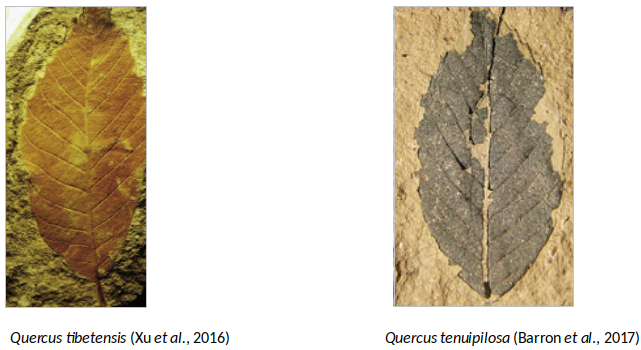
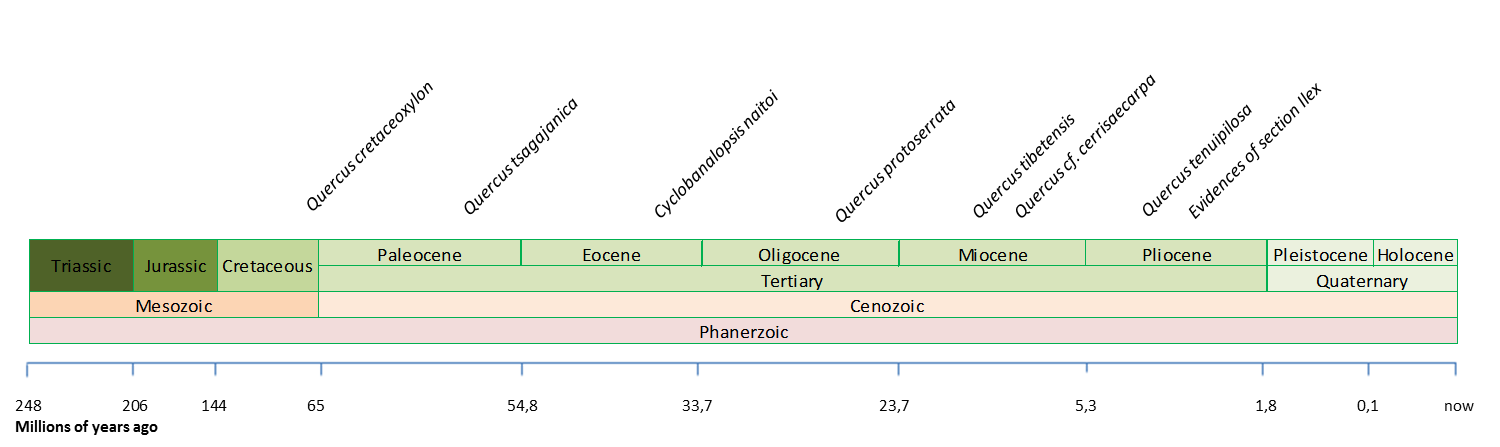
Most important fossils found so far of the Quercus genus in Asia (Barron et al., 2017; Xu et al., 2014; Binh et al., 2018. Illustration by Zamira Betancourt).
America
In general terms, the Paleogene and Neogene fossil record of Quercus is dominated by leaf specimens in western North America, particularly in the Oligocene through Pliocene, and generally by pollen records in eastern North America (Ochoa et al. 2012; Baumgartner 2014).
The earliest evidence acorns of Quercus in North America is Quercus paleocarpa, from the middle Eocene Clarno formation of Oregon from approximately 44 Ma, which is related to the subgenus Cyclobalanopsis. Records in the Eocene (especially leaves) are scanty in North America, in particularly because of the difficulty in distinguishing the features of Quercus from other fagaceous genera. During the middle Eocene, the reproductive structures found of Quercus oligocenensis in Tennessee are also noteworthy. In late Eocene, sections Quercus, Lobatae and Protobalanus as well as some Cyclobalanopsis oaks were present in North America whereas sections of Lobatae and the Cyclobalanopsis occurred in Europe. Alternately the section Protobalanus appeared in the Eocene in North America and seems to be always limited to this continent (Barron et al., 2017).
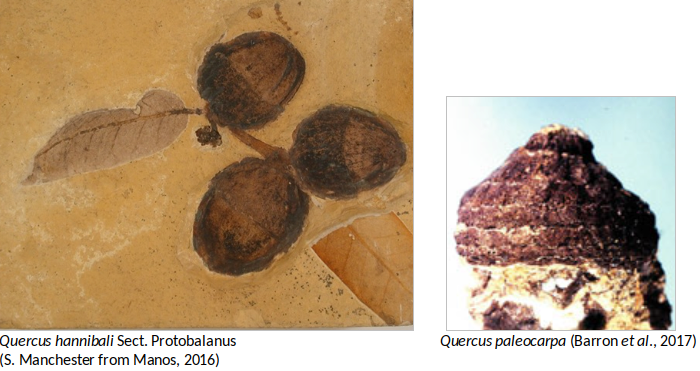
Fossil leaves, wood, pollen, fruits and flowers belonging to Quercus species are common from Oligocene through Quaternary floras of the Northern Hemisphere. Because this abundance of leaves in the fossil record of oaks, identifications are frequently based upon one or a few specimens making it difficult to distinguishing species of closely related extant species of Quercus. For these reasons, the current number of fossil species of oaks is most likely exaggerated, and in need of a serious revision. Remarkably, the only record of the group Cerris in America comes from this time represented by the leaf remains of Quercus prevariabilis (Barron et al., 2017). Acorns from the Miocene flora are common in the West of North America, like Quercus hannibali (Section Protobalanus) from Nevada. Even though these acorns are very common, usually they are not very well preserved making it difficult to identify (Barron et al., 2017). The Neogene (Miocene and Pliocene) record is represented largely by pollen fossils in the eastern North America and shows establishment and expansion of the sections Quercus, Lobatae and Protobalanus.
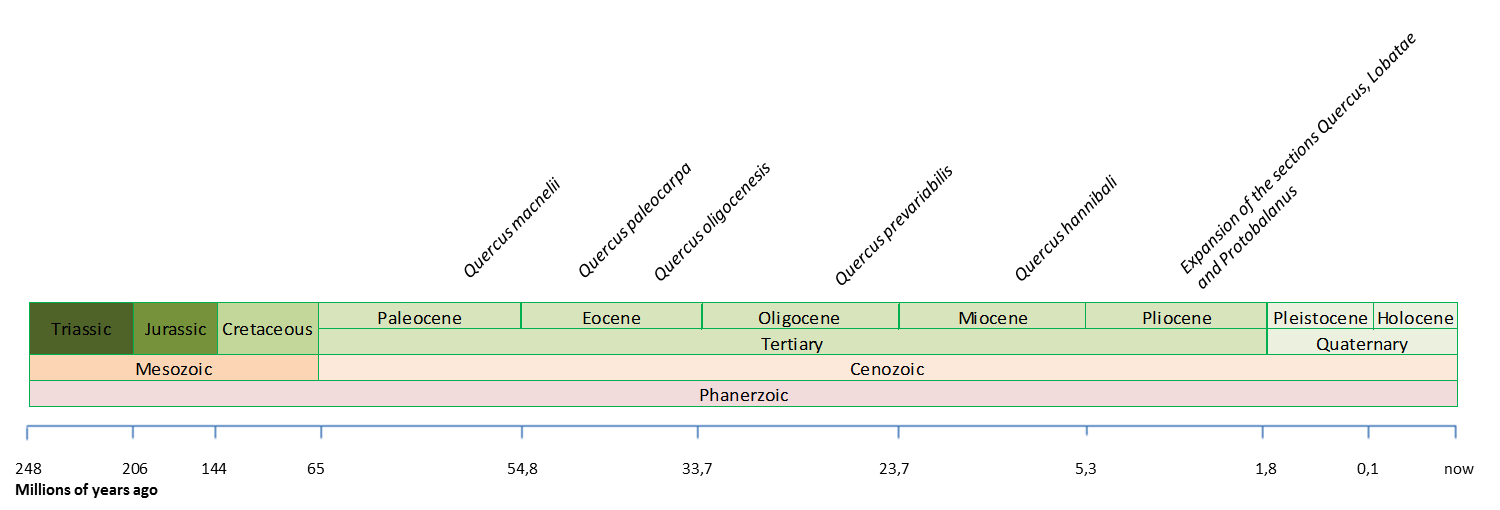
Most important fossils found so far of the Quercus genus in America (Barron et al., 2017; Xu et al., 2014; Binh et al., 2018. Illustration by Zamira Betancourt).
Late Pleistocene Evolutionary Biologyn
During the quaternary era, oaks in the northern hemisphere were subjected to important migrations in response to climatic changes. There were about 17 Milankovitch climate oscillations (alternation of glacial-interglacial periods) during which oak species were subjected to successive restrictions and expansions of their distributions. A glacial period lasted from 50 to 100 thousand years, whereas interglacial periods were much shorter and lasted between 10 to 20 thousand years. Climatic oscillations were strong selective forces favoring species that were agile enough to track their moving habitats (Dynesius and Jansson, 2000). They were most likely responsible for the reduced number of species that occupy large continental distribution (Q. robur in Europe, Q. alba in America, or Q. acutissima in Asia). These movements have profoundly influenced the genetic diversity of the species, but in rather different ways between North America and Europe (Grivet et al., 2006; Kremer et al., 2010). By combining paleobotanical and phylogeographic investigations, migration pathways since the last glacial maximum have been reconstructed in Europe and North America.
In Europe
A large survey conducted in Europe confronting the remaining historical footprints (pollen deposits) to genetic fingerprints (chloroplast DNA (cpDNA) polymorphisms) demonstrated how the extant distribution of genetic diversity was shaped by the dynamics of postglacial colonization (Kremer, 2002). At the end of the last glaciations, European oaks were restricted to three major refugia (Southern Iberian Peninsula, Central Italy, and Southern Balkan Peninsula). As glacial periods lasted more than 100 000 years, species were most likely genetically differentiated among these refugial zones as shown by the completely different haplotype lineages (=cpDNA variant) occupying these regions. In less than 7000 years (from 13 000 to 6 000 BP), oaks recolonized the majority of their modern ranges starting from the refugial areas (Petit et al., 2002a and b; Brewer et al., 2002). Between 13,000 and 10,000 BP oaks increased in abundance in mountainous areas (Pyrénées, South-eastern Alps and Carpathian). The cooling of temperatures during 11,000 BP to 10,000 BP stopped this expansion and resulted in reductions of existing populations. After 10,000 BP, oaks spread throughout Europe and reached their extant distribution at about 6,000 BP. The expansion was more rapid in the west and was reduced in the center and east due to the Alps and the Carpathian mountains.
On average the migration was extremely rapid (between 300 to 500 meters per year) (Brewer et al., 2002). Rare long dispersion events at long distances contributed significantly to the rapid expansion of the species (Le Corre et al, 1997; Davies et al, 2004). These colonization dynamics resulted in a strong phylogeographic structure of the European white oaks, where the maternal lineages of modern oak forest exhibit a strong East-West distribution that witnesses the post glacial migration pathways (see maps).
Colonization dynamics had also contrasting consequences on the genetic diversity of the species. Despite the strong founder effects that accompanied the recolonization, oaks were able to maintain their genetic diversity. Although the highest neutral diversity is restricted to the southern areas of Europe, the level of diversity is still important in the central part of Europe, where the different migration fronts originating from the refugial zones merged (Petit et al., 2002a). However the today’s distribution of adaptive diversity is not correlated to neutral diversity; there is no footprint left by the maternal origin on the variation of adaptive traits (Kremer et al., 2002). Geographic variation for adaptive traits resulted from more recent local selection pressures. Interspecific hybridization was a key migration mechanism as it facilitated the dispersion of late successional species (Q. petraea) into pioneer species (Q. robur). The systematic sharing of the same cpDNA haplotype by different white oak species occupying the same stands indicates that hybridization was extensive during postglacial recolonization (Petit et al., 1997).
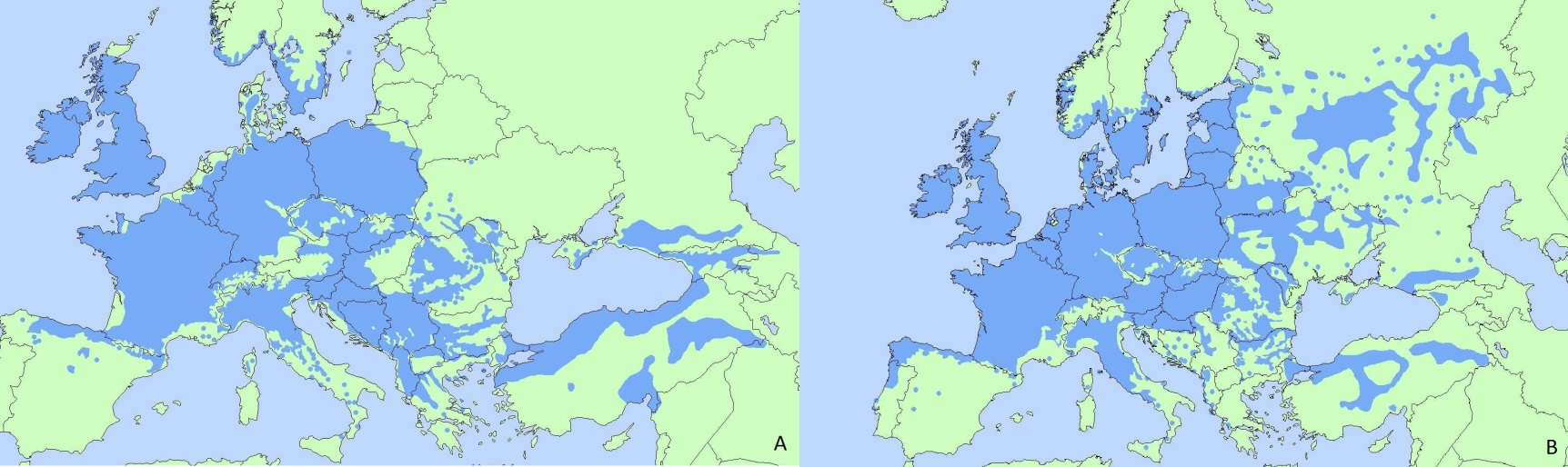
Current European distribution of Quercus petraea (A) and Quercus robur (B). (Image from EUFORGEN).
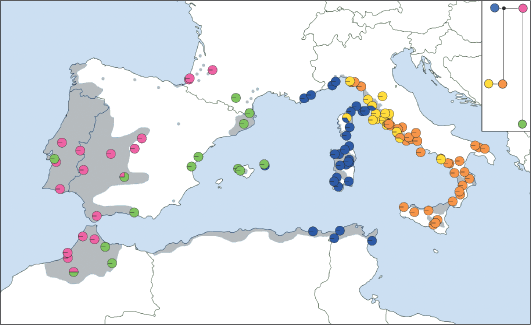
Distribution of cpDNA haplotypes within the populations of Quercus suber and a phylogenetic reconstruction of the relationships among the haplotypes. The grey area corresponds to the modern distribution of Q. suber. (Magri et al., 2007).
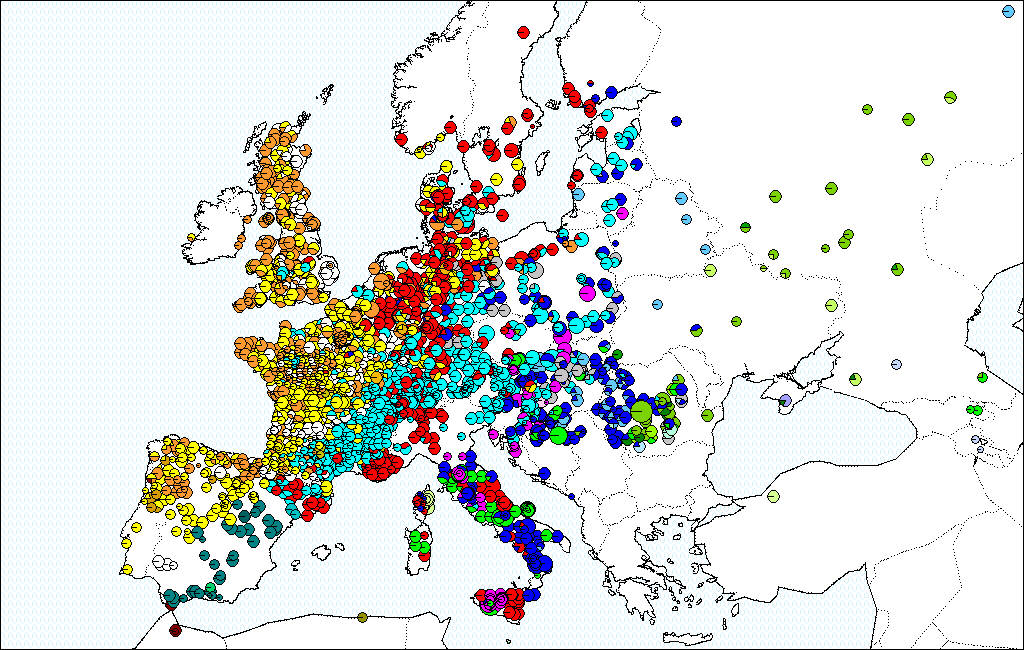
Distribution of cpDNA haplotypes in European white oaks, representing 42 haplotypes in more the 3000 populations (Petit et al., 2002).
In North America
Post glacial colonization dynamics in North America was quite different from Europe. On the Eastern side species were not restricted to genetically separate refugial zones. Furthermore, oak stands persisted as low density populations close to the Laurentide Ice Sheet, reducing opportunities for long distance dispersion and founder events. Hence postglacial recolonization was more diffuse than in Europe and restricted (Schlarbaum et al., 1982). As a result, oaks of eastern North America show less cpDNA differentiation among populations (Magni et al., 2005).
On the western side, oaks were also more stable in response to climatic oscillations. Californian oaks did not become totally extinct during the last glacial period. Populations declined in size, and expanded during the warming periods but by migrating locally, resulting in a patchy distribution of cpDNA diversity, and maintaining larger levels of diversity in comparison to European oaks (Grivet et al., 2006; Dodd and Kashani, 2003). A somewhat similar picture was described in the case of Mediterranean oaks in Spain, suggesting the maintenance of many refugial populations that still persisted today and retain large level of diversity (De Heredia et al, 2007).
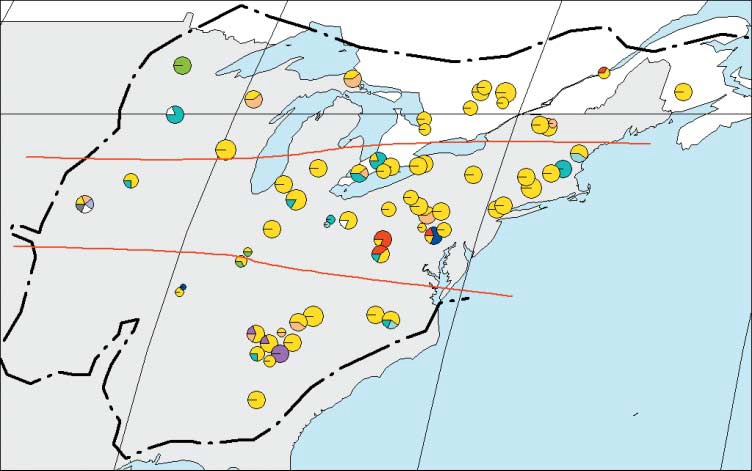
A. Geographic distribution and frequencies of haplotypes in each site throughout the natural range of Quercus rubra (dashed line). Red lines show the three latitudinal subdivisions. B. Colours of the haplotypes and Phylogenetic relationships among haplotypes of Quercus rubra, detected by PCR-RFLP. The numbers indicate the mutation events inferred. (Illustration from Magni et al., 2005).